Lung ultrasound is superior to chest X-ray in the diagnosis and management of pleural effusions. Learn about the identification, differential diagnosis and sizing of pleural effusions using lung ultrasound.

SHARE
TABLE OF CONTENTS
A pleural effusion refers to the pathological accumulation of fluid between the visceral and parietal pleura of the lungs. Under normal conditions, the pleural cavity contains about 10-20 mL of pleural fluid, which lubricates the two pleural layers and maintains a negative pressure during breathing to prevent lung collapse.
Pleural effusions occur due to increased fluid production and decreased lymphatic drainage. Depending on the size and etiology of effusions, they may be asymptomatic, or cause symptoms such as shortness of breath, severe respiratory distress or hypoxia.

Point-of-care ultrasound (POCUS) presents many advantages chest-X ray in the assessment of pleural effusions, especially in an intensive care unit setting. POCUS allows for a rapid, cost-effective and non-invasive lung assessment that can be performed at the patient's bedside without radiation. It can also detect much smaller effusions (around 20 mL) than chest X-rays, which typically only detect 200ml or more of pleural fluid. This difference in sensitivity becomes more pronounced in patients who are lying down as X-rays in these patients often miss large effusions. Ultrasound can also differentiate simple versus complex effusions. This is helpful for determining the cause of the effusion and guiding treatment decisions.
In an ideal situation, pleural effusion evaluation should take place with the patient in a seated position. This allows fluid to collect at the bottom of the lung, making it easier to detect. When a seated position cannot be achieved, place the patient in a supine position with the head of the bed between 30-45 degrees. This is referred to as the “semi-recumbent” position.

The curvilinear and phased array probes are frequently used in lung ultrasound. Operating at low frequencies, these probes excel in providing deep visualization of the pleural cavity, which is needed to accurately assess large effusions. While both probes are suitable for this purpose, the more compact footprint of the phased array transducer stands out for its enhanced maneuverability and ability to get in between the ribs and small spaces. This is especially valuable in an intensive care setting where intricate arrays of patient dressings and medical lines present a unique challenge for image acquisition.
With the probe marker pointing toward the patient’s head, place the probe in the anterior axillary line, at the level of the xiphoid process. If the patient is seated, you can also move the probe posteriorly onto the patient’s back but keep it at the same horizontal level as the xiphoid process.
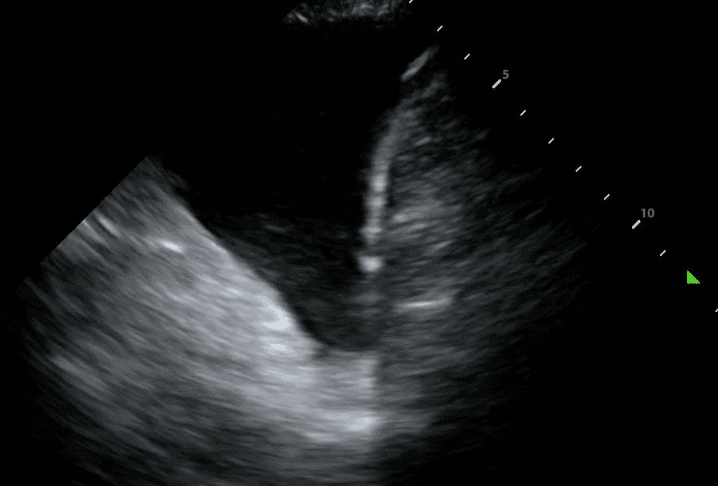
Next, identify the patient’s liver (if your probe is on the right side) or spleen (if your probe is on the left side). Above that, you will see a thin, hyperechoic line which is the patient’s diaphragm.

Finally, above the diaphragm, you will see one one of two things: if there are normal lungs without a pleural effusion, you will see an aerated lung obscuring the diaphragm during inspiration. This is referred to as a “curtain sign.”

If a pleural effusion is present, there will be an anechoic (ie. black) space above the diaphragm. Please note that an anechoic space below the diaphragm represents ascites; not a pleural effusion. Two other other features will stand out to you. Firstly, you will see a “spine sign.” Normally, the spine cannot be visualized above the diaphragm since aerated lung scatters ultrasound beams before it can reach the spine. However, a pleural effusion will allow sound waves to be transmitted to the spine and you will see the spine clearly visible above the diaphragm.

Secondly, you will see a consolidated lung sitting in the effusion. Please refer to our post on consolidations to learn what consolidated lung looks like. If the effusion is large, the consolidated lung will appear to float in the effusion - resembling a jellyfish swimming in the ocean. This is referred to as the “jellyfish sign.”
Estimating the size of a pleural effusion can help determine if, when and where the effusion can be drained. Effusion size may be qualitatively classified as small, medium or large. If there is a “jellyfish sign,” the effusion is at least moderate to large in size.

Many mathematical equations have been proposed to quantify pleural effusions. Specifically, the Goecke 2 formula has shown to be the most reliable yet simple equation at accurately estimating the volume of fluid. This equation measures the total vertical height of the effusion and the distance between the lung base and mid-diaphragm. Unfortunately, this formula requires a patient to be sitting upright which is difficult in an intensive care setting. For this reason, a focus of Deep BreatheTM is to use our large dataset to create deep learning models to reasonably estimate pleural effusion size in supine patients.
The differential diagnosis for pleural effusions is broad and can be narrowed based on whether the effusion is transudative or exudative. This distinction is fundamental in determining the appropriate diagnosis and treatment plan for the effusion.
Transudative effusions are usually caused by conditions associated with increased hydrostatic pressure and decreased oncotic pressure. This pressure gradient decreases lymphatic drainage and pushes more dilute fluid from capillaries into the pleural space. Common causes of transudative effusions include conditions such as congestive heart failure (CHF), hepatic cirrhosis and nephrotic syndrome.
In contrast, exudative effusions are indicative of direct inflammation or injury to the pleura. These effusions are characterized by increased capillary permeability, allowing for a higher concentration of protein, cells and other serum constituents to enter the pleural space. Common causes include pneumonia, hemothorax, cancer, gastrointestinal disease and rheumatic disease.
POCUS can help distinguish between these two types of effusion - a complex effusion on lung ultrasound is almost always exudative while a simple effusion can be exudative or transudative.
Some features of a complex effusion include:
Complex effusions often require drainage for diagnostic and therapeutic purposes.

Some features of a simple effusion include:
In contrast to exudative effusions, a thoracentesis may not be necessary for a transudative effusion unless there is compressive atelectasis or the effusion has not resolved by treating the suspected cause.

In conclusion, lung ultrasound is safer, more portable and superior at identifying pleural effusions compared to chest X-ray, especially in a critical care setting. It allows for real-time evaluation of lung pathology and can distinguish complex versus simple effusions to determine which need thoracentesis. Ultimately, this allows the physician to avoid unnecessary procedures, narrow the differential diagnosis and guide treatment decisions based on specific effusion properties.
References
Arntfield, R. (n.d.). Lung Ultrasound Acquisition Tutorial. Western Sono. Retrieved January 22, 2024, from https://westernsono.ca/screencasts/lung-ultrasound/lung-ultrasound-acquisition-tutorial-2/
Beaudoin, S., & Gonzalez, A. V. (2018). Evaluation of the patient with pleural effusion. Cmaj, 190(10), E291–E295. https://www.cmaj.ca/content/190/10/E291
Bhoil, R., Ahluwalia, A., Chopra, R., Surya, M., & Bhoil, S. (2021). Signs and lines in lung ultrasound. Journal of Ultrasonography, 21(86), 225–233. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8439137/
D’Agostino, H. P., & Edens, M. A. (2021). Physiology, pleural fluid.[Updated 2021 Sep 1]. Stat-Pearls. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK513353/#:~:text=In%20a%20healthy%20human%2C%20the,space%20down%20a%20pressure%20gradient.
Dietrich, C. F., Mathis, G., Blaivas, M., Volpicelli, G., Seibel, A., Wastl, D., Atkinson, N. S. S., Cui, X.-W., Fan, M., & Yi, D. (2016). Lung B-line artefacts and their use. Journal of Thoracic Disease, 8(6), 1356. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4885976/
Hassan, M., Rizk, R., Essam, H., & Abouelnour, A. (2017). Validation of equations for pleural effusion volume estimation by ultrasonography. Journal of Ultrasound, 20, 267–271. https://link.springer.com/article/10.1007/s40477-017-0266-1
Ibitoye, B. O., Idowu, B. M., Ogunrombi, A. B., & Afolabi, B. I. (2018). Ultrasonographic quantification of pleural effusion: comparison of four formulae. Ultrasonography, 37(3), 254. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6044225/
Jany, B., & Welte, T. (2019). Pleural effusion in adults—etiology, diagnosis, and treatment. Deutsches Ärzteblatt International, 116(21), 377. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6647819/
Knipe, H. (2023). Pleural Effusion Volume (Ultrasound). Radiopaedia. https://radiopaedia.org/articles/pleural-effusion-volume-ultrasound
Krishna, R., Antoine, M. H., & Rudrappa, M. (2023). Pleural effusion. In StatPearls [Internet]. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK448189/
Lee, F. C. Y. (2016). Lung ultrasound—a primary survey of the acutely dyspneic patient. Journal of Intensive Care, 4(1), 1–13. https://jintensivecare.biomedcentral.com/articles/10.1186/s40560-016-0180-1
Murali, A., Prakash, A., Dixit, R., Juneja, M., & Kumar, N. (2022). Lung ultrasound for evaluation of dyspnea: a pictorial review. Acute and Critical Care, 37(4), 502–515. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9732207/
Porcel, J. M., & Light, R. W. (2006). Diagnostic approach to pleural effusion in adults. American Family Physician, 73(7), 1211–1220. https://www.aafp.org/pubs/afp/issues/2006/0401/p1211.html
Prina, E., Torres, A., & Carvalho, C. R. R. (2014). Lung ultrasound in the evaluation of pleural effusion. Jornal Brasileiro de Pneumologia, 40, 1–5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4075927/
Shkolnik, B., Judson, M. A., Austin, A., Hu, K., D’Souza, M., Zumbrunn, A., Huggins, J. T., Yucel, R., & Chopra, A. (2020). Diagnostic accuracy of thoracic ultrasonography to differentiate transudative from exudative pleural effusion. Chest, 158(2), 692–697. https://journal.chestnet.org/article/S0012-3692(20)30465-7/abstract
Soni, N. J., Franco, R., Velez, M. I., Schnobrich, D., Dancel, R., Restrepo, M. I., & Mayo, P. H. (2015). Ultrasound in the diagnosis and management of pleural effusions. Journal of Hospital Medicine, 10(12), 811–816. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4715558/
Yu, H. (2011). Management of pleural effusion, empyema, and lung abscess. Seminars in Interventional Radiology, 28(01), 75–86. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3140254/
Zoia, A., & Drigo, M. (2016). Diagnostic value of Light’s criteria and albumin gradient in classifying the pathophysiology of pleural effusion formation in cats. Journal of Feline Medicine and Surgery, 18(8), 666–672. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10816379/
SHARE